CBSE Sample Papers for Class 10 Science Paper 9
These Sample papers are part of CBSE Sample Papers for Class 10 Science. Here we have given CBSE Sample Papers for Class 10 Science Paper 9.
Time allowed : 3 hours
Maximum marks : 80
General Instructions
- The question paper comprises two sections, A and B. You are to attempt both the sections.
- All questions are compulsory.
- All questions of Section-A and B are to be attempted separately.
- There is an internal choice in two questions of three marks each and one question of five marks.
- Question numbers 1 and 2 in Section-A are one mark question. They are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section-A are two marks questions. These are to be answered in 30 words each.
- Question numbers 6 to 15 in Section-A are three marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section-A are 5 mark questions. These are to be answered in 70 words each.
- Question numbers 22 to 27 in Section-B are based on practical skills. Each question is a two marks question. These are to be answered in brief.
SECTION – A
Question. 1.
Why do we feel tired and have pain in muscles and sometimes get cramps after strenous physical work?
Question. 2.
What will happen if intake of Iodine in our diet is low ?
Question. 3.
What is anode mud ?
Question. 4.
Why are ‘danger’ signal lights red in colour ?
Question. 5.
Quote three instances where human intervention saved the forests from destruction.
Question. 6.
On what does the strength of an electromagnet depend ?
OR
Why does a domestic electric circuit have an earth wire ?
Question. 7.
Calculate the amount of heat generated while transferring 90000 coulomb of charge between the two terminals of a battery of 40 V in one hour. Also determine the power expended in the process.
Question. 8.
How does the electronic configuration of an element is related to its position in the periodic table ?
Question. 9.
What is the significance of the word ‘potenz’ in chemistry ? What does it indicate ?
Question. 10.
While cleaning a garden, the gardener gets rid of all insects including earthworms from the soil. Will it have any effect on the soil in the garden ?
OR
Is it sensible to go back to the lifestyle of our grandparents in taking a cloth bag to the shop and purchasing provisions from the nearby grocer shop where he would pack the provisions in paper ?
Question. 11.
After fertilisation, what are the changes inside and outside the ovule ? Is there any part of the flower that remains in the fruit ? If yes, which part and in which fruit ?
Question. 12.
Three students were on the way from Agra to Delhi. Rishi, the one of the student among them who was driving the car saw from his side mirror that the car which was behind their car had met with an accident. He suddenly applied brakes even after his friends asked him to leave the situation as it was. But Rishi didn’t agree and got down of car and was also able to pursuade his friends to help the injured. The three students took the injured person to the nearest hospital. After getting conciousness, the victims please them with thanks for saving their life.
(a) Name the type of mirror from which Rishi saw the accident :
(b) Why this mirror is used as a side mirror in the vehicle ? Show it with the help of ray diagram.
Question. 13.
Is it necessary to mention the physical states of the reactants and products in a chemical equation. Explain with an example.
OR
(a) A dry pallet of common base ‘X’, when kept in open air absorbs moisture and turns sticky. The compound is also a by-product of chlor-alkali process. Identify ‘X’. What type of reaction occurs when ‘X’ is treated with strong acid ? Write a balanced chemical equation for such reaction.
(b) Can we store the base ‘X’ in an aluminium container ? Give reason in support of your answer.
Question. 14.
What is regeneration ? Where do you see it ? Is it similar to reproduction ?
Question. 15.
Compare natural and artificial ecosystem.
Question. 16.
Explain the underlying principle and working of an electric generator by drawing a labelled diagram. What is the function of brushes ?
Question. 17.
Ethanol is a liquid at room temperature. Discuss its physical and chemical properties.
OR
Write the balanced chemical equation for the following :
(a) Methane is burned in sufficient air.
(b) Ethanol is treated with sodium.
(c) Ethanoic acid is reacted with sodium Hydroxide.
(d) Ethanoic acid is treated with sodium carbonate.
(e) Ethanol is mixed with ethanoic acid in the presence of an acid.
Question. 18.
What is meant by double circulation and why is it seen only in birds and mammals ?
Question. 19.
(a) Rohit claims to have obtained an image twice the size of an object with a concave lens. Is he correct ? Give reason for your answer.
(b) Where should an object be placed in case of a convex lens to form an image of same size as of the object ? Show with the help of ray diagram the position and the nature of the image formed.
(c) With the help of ray diagram, illustrate the change in position, nature and size of the image formed if the convex lens in case of (ii) is replaced by concave lens of same focal length.
Question. 20.
How did the Modem periodic table answer many of the queries raised in Mendeleev’s periodic table ?
Question. 21.
What is the importance of wildlife ? Give some measures to conserve our wildlife ? Give some examples of endangered plant and animal species ?
OR
Discuss the role of a food chain in an ecosystem with an example. Comment on the energy flow through a food chain.
SECTION – B
Question. 22.
When an iron rod is dipped into a solution of copper sulphate, copper is displaced. Why is it so ?
Question. 23.
Iron filings were added to a solution of copper sulphate. After 10 minutes, it was observed that the blue colour of the solution changes and a layer gets deposited on iron filings. What is the colour of the solution and the layer deposited ?
Question. 24.
While preparing a temporary stained mount of a leaf epidermal peel, how is the extra stain removed ?
Question. 25.
The given slides A and B were identified by four students I, II, III and IV as stated below :

Question. 26.
A student did the experiment to find the equivalent resistance of two given resistors, Rj and R2 first when they are connected in series and next when they are connected in parallel. The two values of the equivalent resistance obtained by him were Rs and Rp respectively. Compare Rg and RP.
OR
Which of the circuit components in the following circuit diagram are connected in parallel ?

Question. 27.
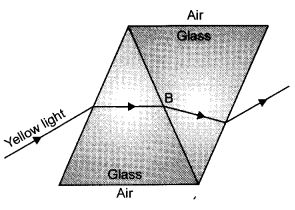
An identical prism is stuck to the first prism using a transparent adhesive with the same refractive index as the glass. This is shown in figure. In this figure, draw the path of the ray after it has reached B and until it has passed into the air again.

ANSWERS
SECTION – A
Answer. 1.
This is due to accumulation of lactic acid in the muscles.
Answer. 2.
- When iodine intake is low, release of thyroxine from thyroid gland will be less by which protein, carbohydrate and fat metabolisms will be affected.
- A person might suffer from goitre in case of iodine deficiency in the body.
Answer. 3.
Anode mud is a deposit of insoluble residue or mixture that collects at the anode in an electrolytic refining process and is formed by the dissolution of the anode in commercial electrolysis or plating process. The soluble impurities go into solution. It is also referred to as the anode slime. Some of the elements present in anode mud are Tellurium, Selenium, Antimony, Gold, Silver, Platinum and copper which are recovered later.
Answer. 4.
Danger signal lights are red in colour because the red coloured light having longer wavelength is scattered the least by fog or smoke. Therefore, it can be seen clearly from a distance.
Answer. 5.
The human intervention that saved the forests from destruction are the following :
- The ‘Chipko Andolan’ (‘Hug the Trees Movement’) in a remote village called Reni in Garhwal, during the early 1970s.
- The sal forsts of Arabari (in West Bengal) were saved from the degradation with the active and willing participation of the local community.
- Amrita Devi Bishnoi, who in 1731 sacrificed her life along with 363 others for the protection of ‘khejri’ trees in Khejrali village near Jodhpur in Rajasthan.
Answer. 6.
The strength of an electromagnet depends on the following factors :
- Number of turns in the coil : If we increase the number of turns in the coil, the strength of electromagnet increases.
- Current flowing in the coil: If the current in the coil is increased, the strength of electromagnet increases.
- Length of air gap between its poles : If we reduce the length of air gap between the poles of an electromagnet, then its strength increass.
OR
Domestic circuits generally have an earth wire which has an insulation of green colour. It is usually connected .to a metal plate deep in the earth near the house. This is a safety measure especially for those appliances that have a metallic body, for example, table fan, toaster, refrigerator etc. The metallic body is connected to the earth-wire which provides a low-resistance conducting path for the current. Thus, it ensures that any leakage of current to the metallic body of the appliance keeps its potential to that of the earth, and the user may not get a severe shock.
Answer. 7.
Here charge transferred Q = 90000 C, potential difference between the terminals of battery V = 40 V and time t – 1 h = 3600 s.
∴ Current, I = \(\frac { Q }{ t } \) = \(\frac { 90000 }{ 3600 } \) = 25A
∵ Amount of heat generated, H = VIt = 40 × 25 × 3600 = 3600000 J = 3.6 × 106 J
and power expended, P = \(\frac { H }{ t } \) = VI = 40 × 25 = 1000 W
Answer. 8.
The electronic configuration of an atom gives us the atomic number of an element as the number of electrons is equal to the number of protons in the atom. The atomic number of an element is a more fundamental property than its atomic mass. The Modem Periodic Table has 18 vertical columns known as groups and 7 horizontal rows known as periods. The elements present in a group have the same number of valence electrons. In other words groups in the Modem Periodic Table signify an identical outer shell electronic configuration. Similarly in a period, the atomic number increases by one unit as we move from left to right. The position of an element in the table tells us about its chemical reactivity.
Answer. 9.
The ‘p’ in pH stands for potenz in German and it means power. The pH scale was developed to measure the hydrogen ion concentration in a solution. On the pH scale we can measure from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower the pH value. The pH of a neutral solution like pure water is 7. Values less than 7 on the pH scale represent acidic solutions. When the pH value increases from 7 to 14 it represents an increase in the OH- ion concentration in the solution meaning an increase in the strength of alkali.
Our body works within a pH range of 7.0 to 7.8. Living organisms survive only in a narrow range of pH change. When the pH of rain water is less than 5.4 it is called acid rain. This affects aquatic life. pH is important in our daily lives. It plays a role in our digestive system, self-defense of animals and plants and in tooth decay.
Answer. 10.
While cleaning his garden if the gardener gets rid of all insects including earthworms he is sure to face a number of problems. Initially, the garden may look neat and insect free but soon there will be a loss of fertility in the soil. This is because the tiny organisms like snails, small beetles and earthworms help in decomposing the dead leaves, flowers or fruits that fall down from the plants. Moreover their waste acts as natural manure for the soil. Next the earthworm helps in moving the soil particles up and down in the soil floor. This helps to improve aeration in the soil. In fact, the earthworm is called the farmer’s friend as it improves soil fertility.
OR
In the name of convenience and a modern lifestyle in the last thirty years mankind has adopted several practices whose harmful effects are becoming visible only now. It has led to problems both to the environment and personally to man himself. Today there are mounds of wastes all around us and in particular plastic so that man does not know how to dispose of them. Moreover, the habit of going in for packed food items everywhere has led to several grave health problems staring at mankind. In those days we purchased provisions in the neighbourhood provision store where we could feel the items with our hands and fingers before buying it and then the grocer would pack everything in old paper bits which we would bring home in our own cloth bags. Thus, there was no plastic in use. Moreover the birds, squirrels and some creatures would feed on the grains that would fall outside the shop. With the advent of packed food items available in supermarkets this has stopped and today sparrows have almost become extinct around us. So it is very sensible to go back to the lifestyle of our grandparents.
Answer. 11.
We know that in a flower, the stamens are the male reproductive part and the carpel is the female reproductive part. The stamen consists of the filament and anther. The anther has many yellow coloured pollen-grains which are the male gametes. The carpel consists of stigma, style and ovary. The female gamete or the ovule is found inside the swollen ovary at the bottom of the carpel.
During pollination, the pollen-grains are transferred to the sticky stigma of the flower. After the pollen lands on a suitable stigma, it has to reach the female gametes inside the ovary. For this a tube grows out of the pollen-grain and travels through the style to reach the ovary. After fertilization, the zygote divides several times to form an embryo within the ovule. The ovule developes a tough outer coat and is gradually converted into a seed. The ovary grows rapidly and ripens to form the fruit. Meanwhile, the sepals, petals, style and stigma shrivel and fall off. Sometimes, the sepals alone remain in the fruit. This is seen in brinjal and chillies.

Answer. 12.
(a) Convex mirror.
(b) A convex mirror always produces an erect, virtual and diminished image and gives a wider view as shown in the following figure.

Answer. 13.
A chemical equation can be made more informative by mentioning the physical states of the reactants and products along with their chemical formulae. The gaseous, liquid, aqueous and solid states of the reactants and products are represented by the notations, (g), (I), (aq) and (s) respectively. The word aqueous is written if the reactant or product as a solution in water. When we use the symbol g with H2O it means in the reaction water is used as steam.
Example : 3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
In the above example, Fe in the solid state reacts with water in steam form and forms iron oxide in solid form and releases hydrogen gas.
OR
(a) ‘X’ is NaOH.
When NaOH is treated with strong acid like HCl, neutralisation reaction occurs and salt is formed.
NaOH + HCl → NaCl + H2O
‘X’ strong acid.
(b) No, ‘X’ cannot be stored in aluminium container because aluminium with a coating of oxide reacts with the NaOH to form a salt and water.

Answer. 14.
Many fully differentiated organisms have the ability to give rise to new individual organisms from their body parts. If the individual is somehow cut or broken into many pieces, many of these pieces grow into separate individuals. Simple animals like Planaria and Hydra, when cut up into pieces, each piece can grow into a new and complete organism. This process is called regeneration. Regeneration is carried out by specialised cells. These cells proliferate and give rise to a large number of cells which in turn undergo specialisation to form the complete organism. These changes take place in an organised sequence referred to as development. However regeneration is not the same as reproduction since most organisms would not normally depend on being cut up to be able to reproduce.

Answer. 15.
Natural ecosystems include forests, ponds, lakes etc. Artificial ecosystems include gardens, crop- fields, an aquarium, national parks, national sanctuaries etc.
Natural ecosystem
- It consists of many species of plants and animals.
- The genetic diversity is very high.
- Sunlight is the energy source for the autotrophs.
- There are long and complex food chains.
- The natural nutrient cycle is highly eff icient.
Artificial or man-made ecosystem
- It consists of fewer or selected species of plants and animals.
- The genetic diversity is low.
- Sunlight is the ultimate source of energy but artificial supplements are added to promote growth.
- Food chains are simple and sometimes incomplete.
- There is incomplete nutrient cycling.
Answer 16.
Electric generator : A labelled diagram of an A.C. electric generator has been drawn in the following figure.

Principle : An electric generator works on the principle of electromagnetic induction phenomenon. According to it, whenever a coil is rotated between the poles of a magnet, an induced current is set up in the coil, whose direction is given by Fleming’s right-hand rule.
Working: Let in the beginning, as shown in figure brushes B1 and B2 are kept pressed separately on rings R1 and R2 respectively. Let the axle attached to the rings is rotated such that arm AB of the coil moves up and arm CD moves down in the magnetic field. Due to rotation of arms AB and CD induced currents are set up in them. As per Fleming’s right-hand rule induced currents in these arms are along the directions AB and CD respectively. Thus, an induced current flows along ABCD and current in the external circuit flows from B2 to B1.
After half a rotation, arm AB starts moving down and the arm CD upward. Therefore, directions of induced currents in these arms change. Thus, net induced current now becomes in the direction DCBA. In the external circuit now current flows from B1 to B2. Thus, after every half rotation current changes its direction and an alternating current is obtained from the generator.
Action of brushes : Brushes are kept pressed on the two slip rings separately. Outer ends of the brushes are connected to the galvanometer (or the external load). Thus, brushes help in transferring current from the coil ABCD to the external circuit.
Answer. 17.
Ethanol (C2H5OH) is a commercially important hydrocarbon.
Physical properties of ethanol :
- It is a liquid at room temperature.
- It is commonly called alcohol and is an active ingredient of all alcoholic drinks.
- Being a good solvent, it is used in medicines like cough syrup, tonics and tincture iodine.
- Consumption of even a small amount of pure alcohol called absolute alcohol can be deadly.
- Consumption of alcoholic drinks is a social problem and it leads to many harmful effects.
Chemical properties of ethanol :
(i) Reaction with sodium: Alcohols react with sodium leading to the evolution of hydrogen. Sodium ethoxide is formed when sodium reacts with ethanol.
2Na + 2C2H5OH → 2C2H5ONa + H2
(ii) Reaction on heating: Heating ethanol at 443K with excess concentrated sulphuric acid results in the dehydration of ethanol to produce ethane.
![]()
As concentrated sulphuric acid removes water molecule from ethanol it is called a dehydrating agent.
OR
(a) CH4 + 5O2 → CO2 + H2O + Heat + Light
(b) 2CH3CH2 – OH + 2Na → 2C2H5 – ONa + H2
(c) CH3COOH + NaOH → CH3COONa + H2O
(d) CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
(e) CH3COOH + CH3 – CH2 – OH → CH3COOCH2 – CH3 + H2O
Answer. 18.
Double circulation refers to the separate circulation of pure oxygenated blood from the heart to different body parts and the impure deoxygenated blood collected from the different body parts and sent to the heart. This separation allows a highly efficient supply of oxygen to the body. In double circulation blood goes through the heart twice during each cycle. In fact the separation of the right side and the left side of the heart helps to keep the oxygenated and deoxygenated blood from mixing. Animals like birds and mammals which require a lot of energy daily to maintain their body temperature have double circulation. Animals like amphibians and reptiles have a three chambered heart which allows some mixing of blood. They do not require a lot of energy as their body temperature depends on the surrounding temperature. Fishes have only a two-chambered heart. Blood pumped to the gills, is oxygenated there and is sent directly to the rest of the body. Thus blood goes only once through the heart in the fish during one cycle of passage through the body.
Answer. 19.
(a) No, Rohit is incorrect because magnified imag6 of an object cannot be formed by a concave lens ever.
(b) The object should be placed at 2F.


Image obtained is virtual, erect and diminished in case of concave lens.
Answer. 20.
The Modem Periodic Table was able to answer many of the queries posed by Mendeleev’s periodic table. Clear trends could be seen among the groups and periods with regard to some major issues like valency, atomic size and metallic and non-metallic properties.
Atomic size: Atomic size refers to the radius of the atom. It can be visualised as the distance between the centre of the nucleus and the outermost shell of an isolated atom. The atomic radius of hydrogen atom is 37 picometre.
As we move from left to right along a period, the atomic radius decreases. This is due to an increase in nuclear charge which tends to pull the electrons closer to the nucleus and reduces the size of the atom.
For example, elements of period II, Li(152), Be(lll), B(88), C(77), N(74), 0(66) we can see how the atomic radius gradually decreases in size.
As we move down the group, the atomic size increases. This is because new shells are being added as we go down the group. This increases the distance between the outermost electrons and the nucleus so that the atomic size increases in spite of the increase in nuclear charge.
For example, Group I elements are Na (86), Li(152), K(231), Rb (244), Cs(262).
Such trends can be observed in all the groups and periods of the Modem Periodic Table.
Answer. 21.
Importance of wildlife are :
- They maintain an ecological balance of nature.
- It provides a great biological diversity.
- Many valuable products like musk, ivory, leather etc. are obtained from them.
Some measures to conserve wild life are :
- Implementation of Laws to give severe punishment to those people who kill wild animals.
- Protection of their natural habitats.
- Maintenance of wildlife in protected areas like sanctuaries, national parks, biosphere reserves etc.
- Educating people about the importance of wildlife.
Examples of endangered plant species are Pitcher plant, Snow Orchid and examples of endangered animal species are Indian salamander, Indian wolf, One-homed rhinocerous etc.
OR
Grass → Insects → Frog → Snake → Peacock
The above sequence represents a food chain operating in an ecosystem. The series of organisms which eat and are eaten by others is called a food chain. Each level of the food chain is called a trophic level. Generally the autotrophs or producers are the first trophic level. They trap the solar energy and make it available for the heterotrophs or consumers. The herbivores or the primary consumers come at the second level, small carnivores or secondary consumers come at the third level and larger carnivores or tertiary consumers come at the fourth trophic level. Food forms the source of energy for each level of organism and helps it to perform the various functions of life. There is a flow of energy from one trophic level to another. Solar energy is trapped by the autotrophs during photosynthesis. This energy flows from one trophic level to the next. It has been found that only 1/1 Oth of the energy is capable of moving to the next level. That is why as we move up the food chain we find fewer and fewer organisms. Also every food chain does not have more than three to four levels. The length and complexity of the food chains vary greatly. Each organism is generally eaten by two or more organisms which in turn are eaten by several others. So in any ecosystem, instead of a single straight food chain we can see many branching chains forming a food web.
SECTION-B
Answer. 22.
When an iron rod is dipped into a solution of copper sulphate, copper is displaced because iron is more reactive than copper. So, it displaces copper and forms iron sulphate.
Answer. 23.
The solution of the colour is green and that of the coating is reddish-brown. This is because iron being more reactive than Cu displaces Cu from CuSO4 and forms green coloured solution of FeSO4 and a reddish-brown layer of Cu get deposited.
Answer. 24.
While preparing a temporary stained mount of a leaf epidermal peel, extra stain is removed with the help of the filter paper. The filter paper absorbs the extra starch.
Answer. 25.
I is correct because in binary fission of Amoeba, nucleus divides first, then the cytoplasm and daughter cells are formed.
Answer. 26.
In series, the equivalent resistance
Rs = R1 + R2
In parallel, the equivalent resistance

OR
R1, R2 and V are connected in parallel. This is because these circuit components have two common points.
Answer. 27.
We hope the CBSE Sample Papers for Class 10 Science paper 9 help you. If you have any query regarding CBSE Sample Papers for Class 10 Science paper 9, drop a comment below and we will get back to you at the earliest.
